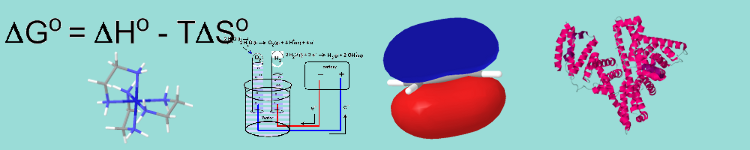
ch231hchapter7
Chapter 7: Thermodynamics
Chapter Learning Goals:
- You know several definitions:
- Energy: the capacity to do work.
- Work: Action of a force through a distance.
- Heat: energy transferred between a system and its surroundings due to temperature differences.
- You know defitions for potential energy and kinetic energy.
- You know that thermal energy is associated with the kinetic energy of molecules in a system.
- You can calculate the quantity of heat transfered between substances if given masses, specific heats and the temperature changes.
- You can predict ΔT for heat transfer between substances given masses, specific heats and initial temperatures.
- You know the First Law of Thermodynamics: energy is neither created nor destroyed, so the energy of an isolated system is constant.
- You recognize that a change in an isolated system can manifest as either work (w) or heat (q), and that ΔU = q + w. For an isolated system, the First Law says ΔUisolated system = 0, so for any change, w = -q.
- For systems interacting with their surroundings, you know sign conventions: if heat is absorbed by the system, q > 0; if heat is given off, q < 0. if work is done on the system, w > 0; if work is done by the system on the surroundings, w < 0.
- You understand that if we control a system to maintain a constant volume, no work is done in any change and thus ΔUsystem = qV. An example is a reaction performed in a sealed bomb calorimeter.
- You know how to define enthalpy H = U + PV, and can articulate why ΔH = qP for a process at constant temperature and pressure.
- You recognize that changes of state are accompanied by specific molar ethalpies: ΔHv for vaporization/condensation, ΔHm for melting/freezing. You know the signs expected for these values.
- You can use Hess's Law to arrive at a net ΔHº given a sequence of processes with known ΔHº that get from the initial state to the final state.
- You know that the ΔHfº for any element in its standard state is 0, and serves as a reference for comparing ΔHfº for any chemical compound.
- You can calculate the standard enthalpy of reaction for any chemical transformation, if given the standard enthalpy of formation for each reactant and each product.
Links:
Brownian motion of nanoparticles (YouTube)